 Installation of Cleanrooms in the Pharmaceutical Industry
Installation of Cleanrooms in the Pharmaceutical Industry
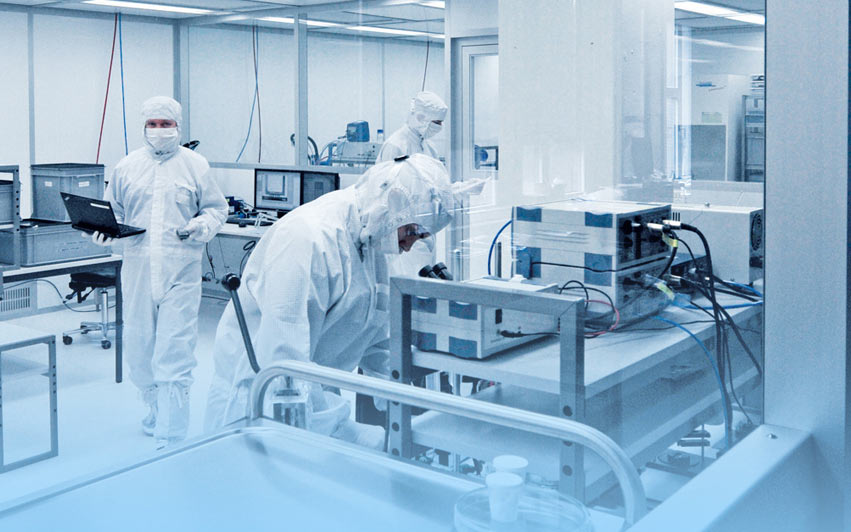
Cleanrooms are a key element in the pharmaceutical industry, where strict hygiene standards are essential to ensure the quality and safety of products. In these sterile environments, the production of medicines, laboratory analyses, research, and development take place, making precision in the installation and operation of these rooms crucial.
The Importance of Cleanrooms in the Pharmaceutical Industry
Cleanrooms provide a controlled environment where particle concentration in the air, temperature, humidity, and pressure are precisely regulated. These rooms are designed to prevent contamination by microorganisms, dust, and other particles, which is especially important in the production of sensitive pharmaceutical products such as injectable drugs, biological agents, and sterile solutions.
Cleanroom Installation Process
The installation of cleanrooms in the pharmaceutical industry requires the collaboration of experts from various fields, including engineers, builders, and specialists in ventilation, filtration, and automation. The installation process involves several key steps:
- Planning and preparation of the space: The installation of cleanrooms begins with thorough planning. It is necessary to carefully review the requirements regarding cleanliness levels (e.g., ISO class), airflow, temperature, relative humidity, and pressure. Additionally, the location of the rooms within the pharmaceutical facility is important, as they must be accessible but separate from areas prone to contamination.
- Material selection: The walls, ceilings, and floors of cleanrooms must be made of smooth, non-porous, and chemically resistant materials that allow easy cleaning and disinfection. Special coatings such as epoxy resins or PVC materials are commonly used, which do not emit particles and prevent dust accumulation.
- Installation of ventilation and filtration systems: Ventilation is a key part of cleanrooms. HEPA (High-Efficiency Particulate Air) filters remove microparticles from the air, maintaining the required level of air cleanliness. The system must ensure adequate airflow to expel contaminated air from the room while providing a constant supply of fresh, filtered air.
- Installation of environmental control systems: Sensors are installed in cleanrooms to precisely measure and control temperature, humidity, and pressure. Automated systems allow continuous monitoring and adjustment of these parameters, ensuring that conditions remain compliant with standards at all times.
- Airtight doors and passages: To prevent air leakage and contaminant intrusion, airtight doors and passages must be installed. Airlocks are often used, allowing safe entry and exit from cleanrooms without the risk of contamination.
- Ensuring antistatic properties: In pharmaceutical cleanrooms, antistatic surfaces are also essential to prevent electrostatic charges, as these could affect production quality or even damage sensitive materials.
Testing and Validation
Once cleanroom installation is complete, the testing and validation process follows. This includes measuring air cleanliness, checking the operation of ventilation systems, testing pressure, temperature, and humidity, and verifying the room's tightness. Only upon successfully passing these tests is the room certified for use in pharmaceutical production.
Maintenance and Regular Inspections
After successful installation and validation, regular maintenance of cleanrooms is essential. This includes cleaning, sterilization, filter replacement, inspection of ventilation systems, and monitoring of environmental parameters. Regular inspections and calibrations ensure that the rooms maintain the required level of cleanliness and operate flawlessly.
Contact Us
If you would like more information about our services, contact us through the contact form. Our team will be happy to listen to you and provide solutions that meet your needs.







